Abstract
Natural killer (NK) cells can have potent anti-tumour responses and recently highlighted potential for memory-like functions, but the best approach to generate memory-like NK (mlNK) cells remains unclear. Priming of NK cells with the pharmaceutical-grade, replication incompetent tumour cell product, INKmuneTM generates tumour-induced mlNK (TIML-NK) cells with enhanced cytokine production and cytotoxicity against multiple NK-resistant tumour target cell lines in vitro, analogous to previously reported cytokine-primed memory-like NK cells. In addition, proteomic profiling of TIML-NK cells revealed differential abundance of proteins involved in promoting mitochondrial survival and function as well as critical nutrient receptors, which may provide a unique benefit of NK cell activation whilst sparing mitochondrial damage typically associated with cytokine-mediated activation. INKmune priming increased NK glycolysis and oxidative phosphorylation whilst enhancing mitochondrial respiratory capacity and maintaining glycolytic reserve.
Four patients have been treated with a cycle of three, weekly infusions of 1x108 INKmuneTM cells. All patients received all three doses without incident and without side effects.
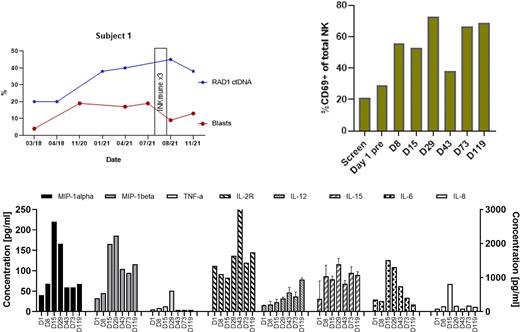
Patient 1 was a 78yr old man with A 3yr history of refractory MLD MDS who was transfusion and platelet dependent and required G-CSF. Within 7 days of first infusion, over 50% of his peripheral blood NK cells activated and this increased with each dose. By day +29, 72% of his NK cells were activated and this remained above 68% at day +119 when monitoring ended. His ECOG status fell from 2 pre-treatment to 0 at D+119 and his RAD-1 ctDNA from 45 to 38. Analysis of systemic cytokines showed increases in MIP-1a/b, TNF-a and sIL2R which paralleled the changes in percentages of CD69+ NK cells. IL6 levels peaked after the second infusion but levels remained low and there was no evidence of CRS. At one year follow-up he remained well, platelet and G-CSF independent, with reduced transfusion requirements and had resumed playing sport with friends.
Subjects 2, 3 and 4, were all multiply relapsed and refractory AML patients; two of whom had relapsed after HLA-mismatched HSCT. All showed rapid activation of peripheral NK cells post infusion with sustained activity throughout follow-up. In all cases these activated NK cells killed NK-resistant tumour cells in vitro without additional activation.
Subject 2 was a neutropenic 21yr old female with 70% a mixed chimera and refractory AML (M2) post VUD HSCT at time of treatment. Within 1 month of initiation of INKmune treatment she was discharged with PMN >500 and her mixed chimera resolved to full donor chimera. At Day 140 post INKmune her bone marrow NK cells remained highly activated (>60% CD69+, K562 and Raji lysis of >70%). Her AML recurred 4 months post INKmune infusion and she died from relapsed disease 8 months after INKmune treatment.
Subject 3 was a 21yr old male with refractory AML (M6) after two failed HSCT (haplo m/m and VUD). He showed the same rapid generation of activated NK cells in vivo but to a lesser degree (maximum 35% CD69+, sustained increased lysis of K562 but no lysis of Raji cells) and no evidence of clinical improvement.
Subject 4, a multiply relapsed AML patient has just been treated and has shown peripheral NK proliferation and maturation at D+8. The subject experienced no adverse effects post 1st and 2nd doses and further follow-up will be presented.
INKmuneTM infusions proved safe and led to rapid and sustained activation of peripheral NK cells with concomitant increases in relevant systemic cytokines. The TIML-NK have a distinct phenotypic, proteomic and metabolomic signature which mirrors the activity in vitro and in vivo.
This is the first time memory-like NK cells have been generated in vivo without the need for cytokine induction or support and our findings inform the development of more optimal NK cell-based immunotherapeutic strategies for cancer.
Disclosures
Sabry:Astrazeneca Ltd: Current Employment. Lowdell:Avectas Ltd: Consultancy; Sartorius GmbH: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal